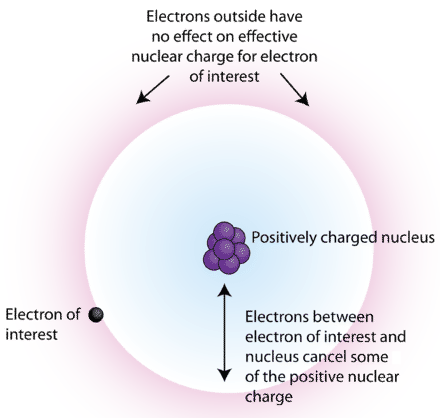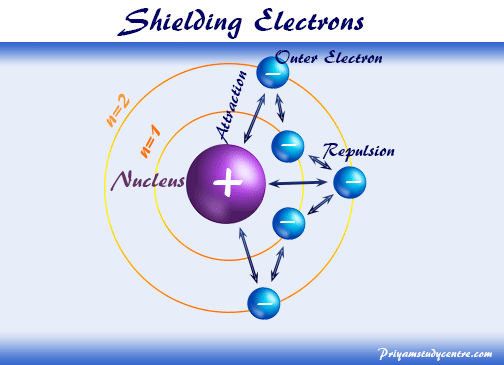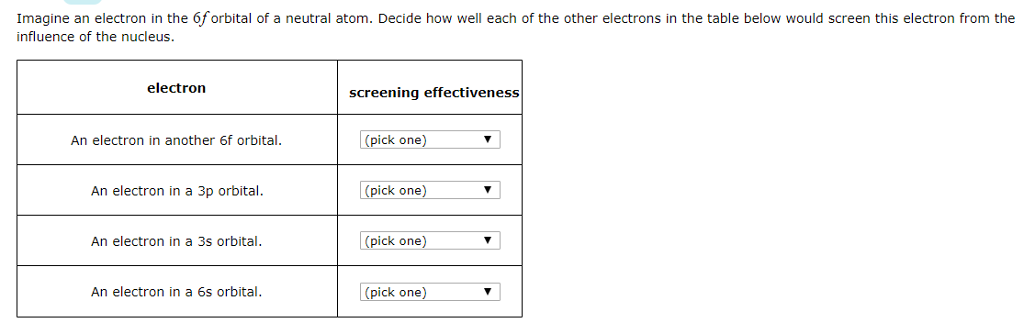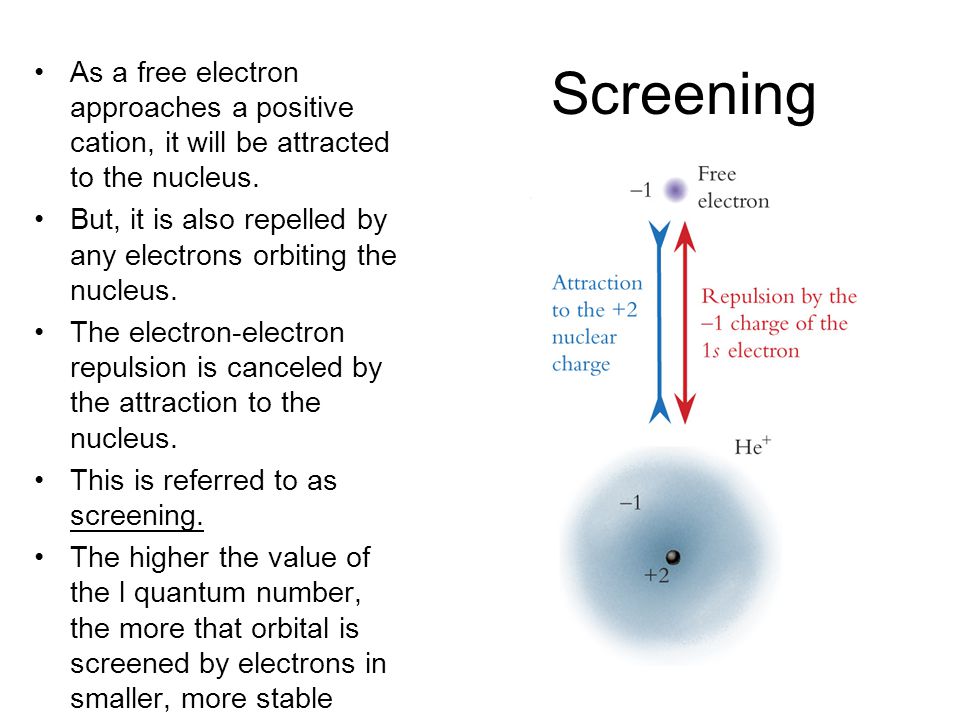
Screening As a free electron approaches a positive cation, it will be attracted to the nucleus. But, it is also repelled by any electrons orbiting the. - ppt download

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram
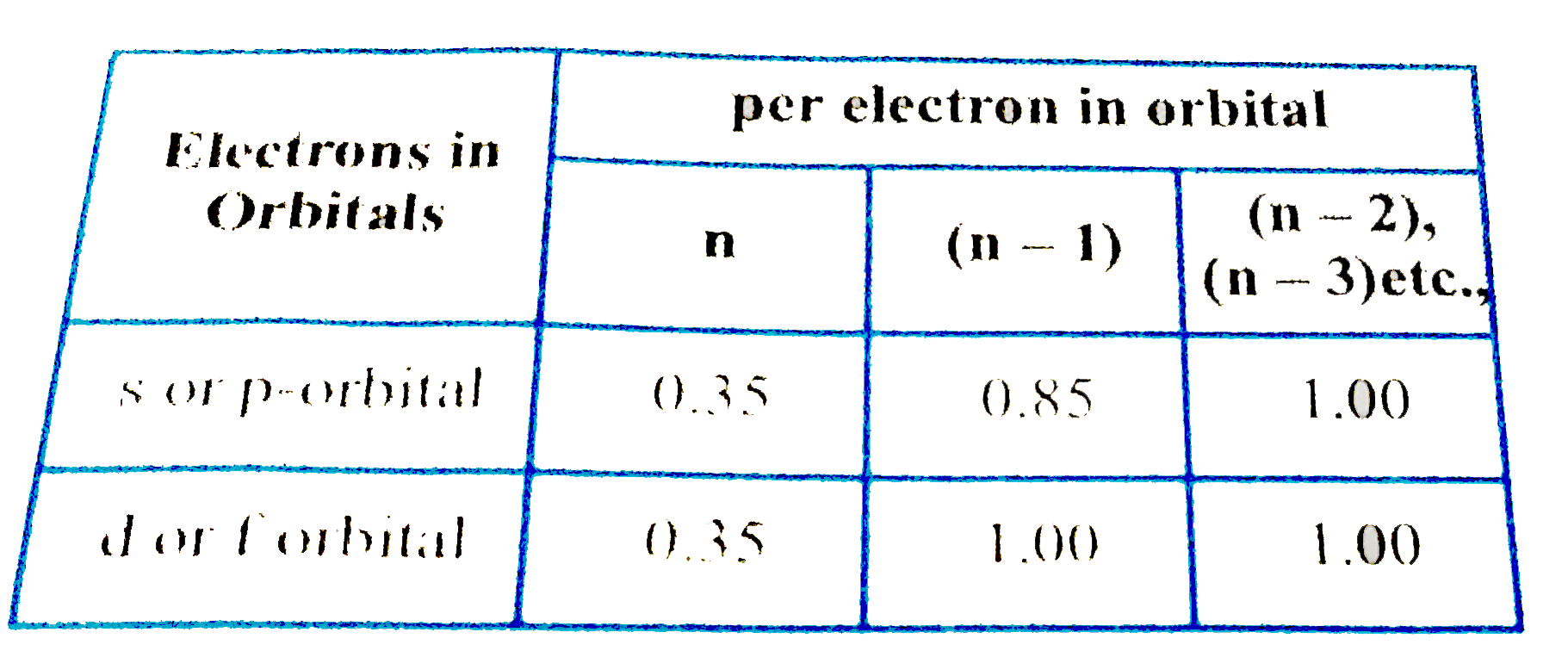
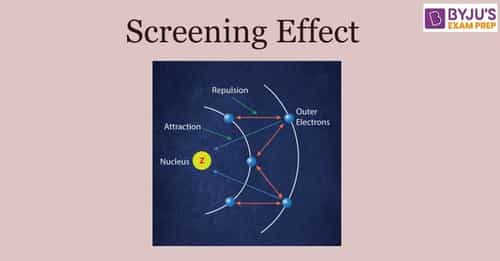
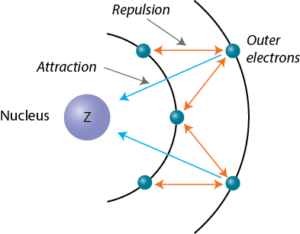
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram

SOLVED: Question 37 (1 point) Screening of the nuclear charge by core electrons in atoms is: less efficient than by valence electrons more efficient than by valence electrons essentially identical to that
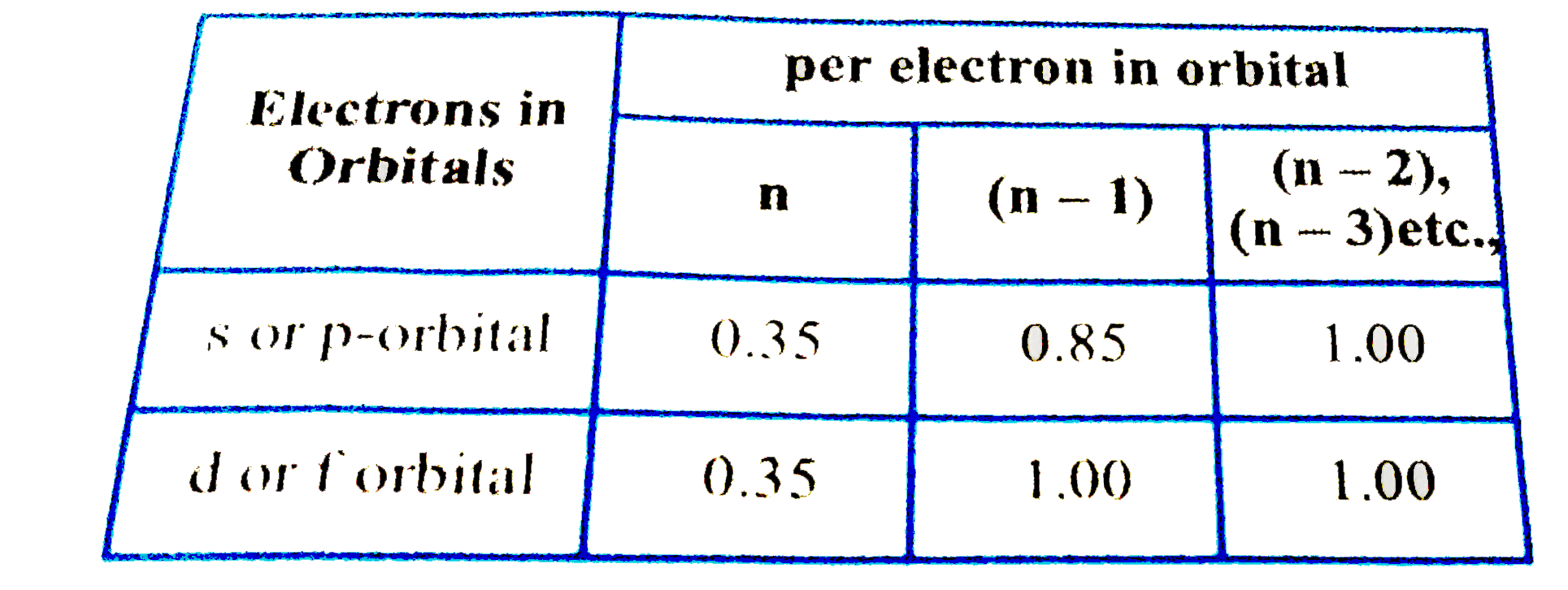
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type

Repulsive electron-electron interaction and nuclear charge screening: Ground state of two-electron atoms: Ndinya, Boniface, Akeyo, Joseph: 9783846540688: Amazon.com: Books
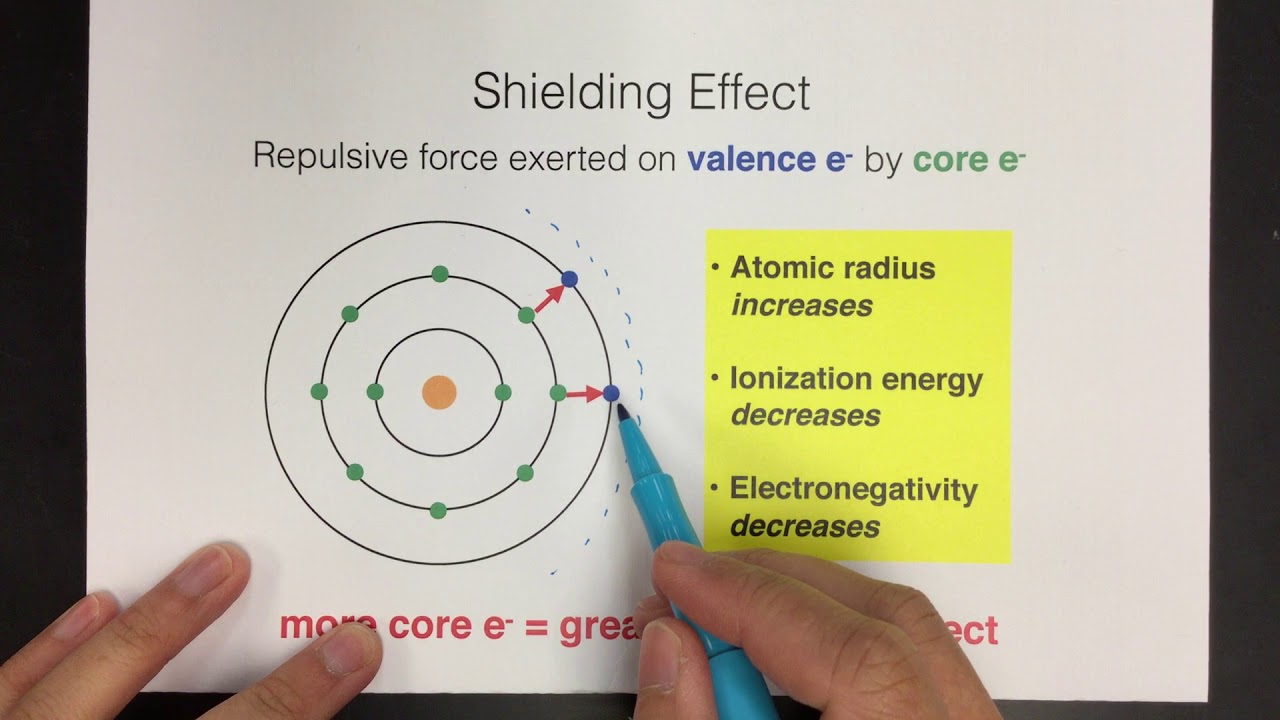
Welcome to Chem Zipper.com......: Effective Nuclear charge (Z* or Zeff): Slater's rule: Screening effect or Shielding effect
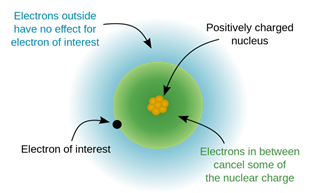
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is: